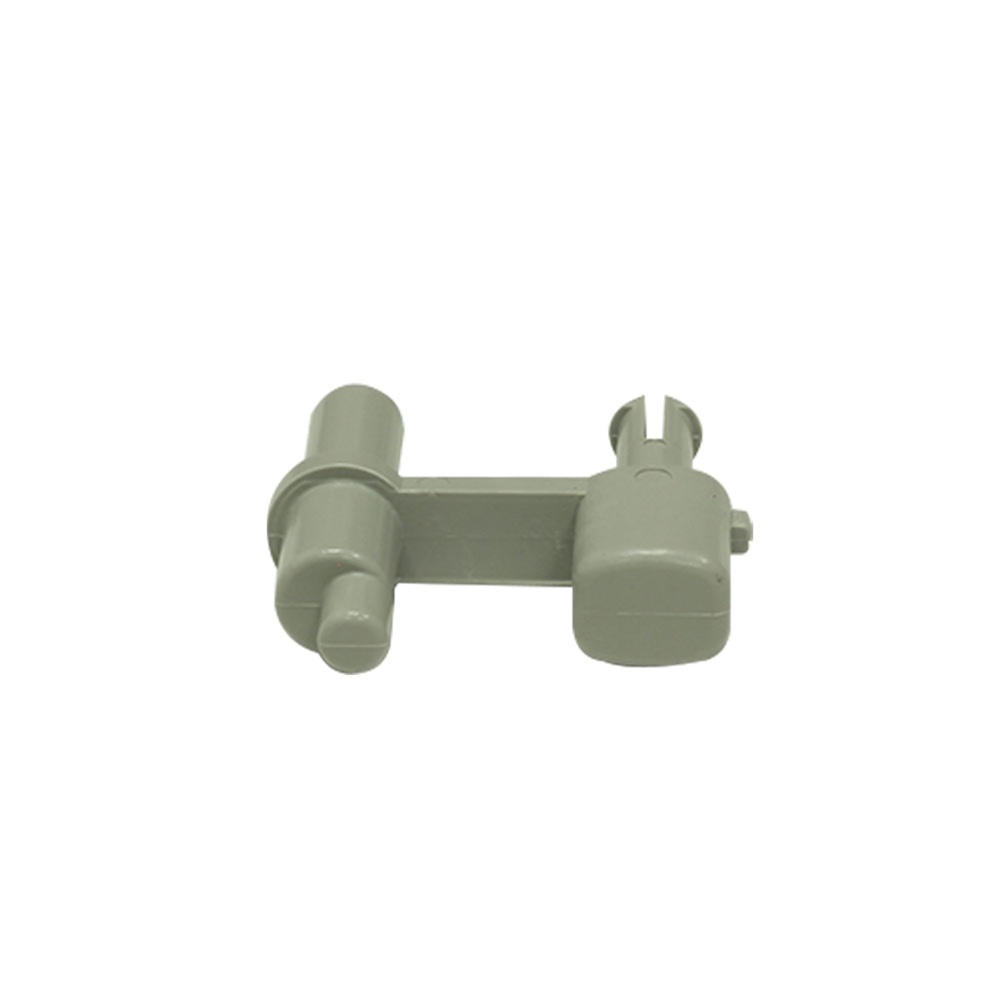
Door Release - Injection Molded
| 6600-2069-500 | |
| New | |
| Maternal Infant Care | |
| MIC S&A Other | |
| GE HealthCare | |
| GE HealthCare | |
| N/A | |
Enter your approval number and submit to add item(s) to cart.
Please enter approval number
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without the
approval number, GE will contact you before your order
can be confirmed for shipment.
Select your approver's name and submit to add item(s) to your cart
Please Select Approver Name
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without
selecting an approver, GE will contact you before your order
can be confirmed for shipment.
Product Overview
The Door Release - Injection Molded is a door release that is used in most of the Maternal Infant Care (MIC) incubators and warmers of the Neonatal Intensive Care Unit (NICU). It almost comes in all the machines, which have a bed in it. This part is one of the accessories of the bed assembly that comes in the side panel. It helps in locking the door of the side panel. A similar part will be used for the same purpose on the rear side of the panel. This will be fixed while assembling the bed arrangement. The door release is manufactured by polymerization of formaldehyde. It is made up of high quality material, which has very good mechanical and insulating properties, and therefore is used for many electrical applications. Injection molding is the method used to fabricating this part. They come with a smooth finish, without any burrs, sharp edges, surface blemishes, sink marks and flash marks. It has a label with the product details, environment label for packaging and a Quality Assurance (QA) seal. It is being used in machines such as Giraffe™ Warmer, Panda™ iRES Warmer and Panda™ Wall Mount Warmer, among others.